GMP monitoring in compliance with 21 CFR Part 11
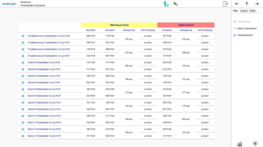
Neuberger’s GMP monitoring system uses ProGrafNT building and process control software. It enables reliable and GMP/FDA-compliant monitoring of clean room processes. All quality-relevant measured values are recorded and archived in a tamper-resistant database. If limit values are exceeded, the monitoring system triggers precisely defined warning and alarm messages. ProGrafNT is well known for its intuitive and convenient operating concept.
Audit trail
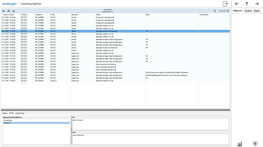
All user interventions remain fully traceable for many years. ProGrafNT permanently records which user changes or enters data, along with the time and location. All previous and subsequent states are recorded with a reason for the change and are stored in the tamper-resistant database. This ensures that no data is lost.
Electronic records
All measured values, states, limit values, alarms and the audit trail are stored in a tamper-resistant SQL database and displayed as freely configurable graphs and tables. It is possible to export the data or transfer it to other systems (e.g. Historian) to produce further evaluations, visual displays or reports.
Trending, alarming
ProGrafNT archives measured values, status values, limit values, messages, etc. in an SQL database. All data is available for creating visual displays in the form of evaluations, reports, etc. at any time.
Plant visualisation
ProGrafNT is notable for its intuitive and convenient operating concept. Visual displays are created according to the customer’s requirements.
User management
The convenient user administration satisfies the GMP requirements set out in FDA 21 CFR Part 11. Password aging, locking in the event of an error message, specification of minimum length, password administration, including via active directory etc., are unrestricted.
Data security
Secure storage of data in the SQL database. Automatic backup directly to network drive or other storage devices. Option of data transfer to higher-level systems (e.g. Historian, etc.).
Sensors
Temperature, humidity, pressure, particle count or flow – the pharmaceutical sector, and clean room environments in particular, are subject to strict requirements in terms of the accuracy and stability of recorded measured values. It is also extremely important that the sensors are resistant to chemicals and cleaning agents. This is why Neuberger uses only high-quality sensor equipment that is installed and qualified by its own professional service engineers.
Create an intelligent combination of GMP monitoring and building services!
If the measured values from the clean room sensors are used for GMP monitoring and for the building management system, this eliminates the need for costly duplicate instrumentation and means that there is no deviation in the recorded measured values.
To ensure the success of your project, Neuberger is always on hand with a solution-focused approach from detailed design through to project completion.
Clean room terminals
Neuberger’s clean room terminals are powerful display, operating and visual display units used primarily in clean rooms and laboratories. They can display current measurement data relating to room conditions, process values and alarm messages and enable operators to make quick decisions on-site. Monitoring and control options for airlocks, doors, light and disinfection are available. The measuring device is calibrated quickly in the installed state.
The size, function and assignment of each panel can be customised to suit individual requirements.