Clean room automation by Neuberger.
For qualified, safe operation.
End-to-end monitoring of processes in clean rooms protects people and products from contamination and ensures that companies in the pharmaceutical, biotechnology, semiconductor and electronics industries do not suffer financially as a result of faulty batches. Neuberger’s monitoring system designed in accordance with GMP and GAMP specifications provides customers with a powerful tool for GMP- and FDA-compliant quality monitoring in clean rooms. The system has been qualified and validated many times over by industry leaders. We will be happy to help you to prepare the documents and assist with the qualification and validation process.
Information about GMP monitoring and clean room solutions (PDF, EN)
GMP hardware and software.
For clean rooms and healthcare.
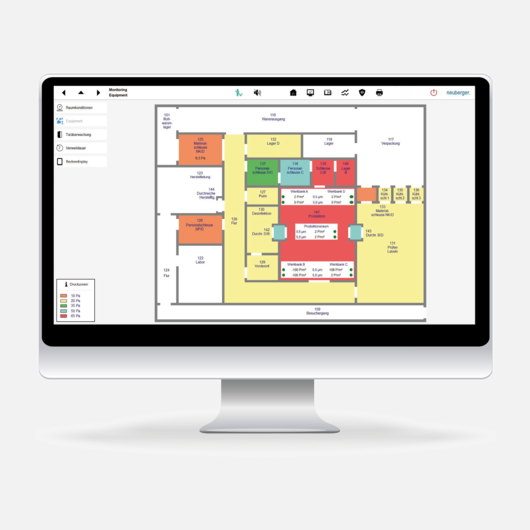
The continuous recording, storage and monitoring of process values such as room temperatures, air humidity or airborne particles is carried out with the powerful GMP monitoring system ProGrafNT. The system ensures tamper-resistant recording and storage of all plant states. The system complies with all regulations in FDA 21 CFR Part 11 pertaining to electronic signatures. Neuberger uses only high-quality sensor technologies to handle the stricter requirements that apply when recording measured values in the pharmaceutical industry, and the clean room sector in particular. Additional clean room terminals are available for operation, visual display and monitoring.
GMP monitoring system
The systems are developed in accordance with GMP and satisfy all requirements set out in FDA 21 CFR Part 11. Our customers can be sure that an external audit will be successful.
Sensors
Sensor equipment for every application, tailored to customer requirements!
Clean room terminals
For the operation, visualisation and monitoring of clean rooms.
GMP services.
From a single source.
In keeping with the company slogan, “A one-stop source for all your needs”, Neuberger provides services including mapping, calibration and qualification. When it comes to mapping, for example, Neuberger’s trained engineers calculate the temperature distribution in storage, refrigeration and/or freezer areas. We then work together to define measures to avoid cold spots and hot spots. We also use certified and tested measuring equipment to perform on-site calibration according to your specifications. As a matter of course, Neuberger also helps with every step of the qualification process, for example preparing professional documentation in accordance with current GMP requirements. Neuberger is your experienced and reliable partner for GMP services.
Mapping
Ensure product quality in storage, refrigeration and freezer areas by calculating temperature distribution and predicting cold spots and hot spots.
Calibration
Calibration on-site or in the factory, always with state-of-the-art measurement technology.
Validation and qualification
Preparation of documentation and completion of qualification process.
Regulation and control.
Intuitive and flexible.
Reliable control and monitoring of personnel and material airlocks is an indispensable component of a GMP-compliant environment. Neuberger’s flexibly configurable PMC programmable logic controllers are integrated perfectly into the central GMP monitoring system. They perform all the main functions of airlock control such as door control, door monitoring, pressure monitoring, alarm triggering, state display and access control.
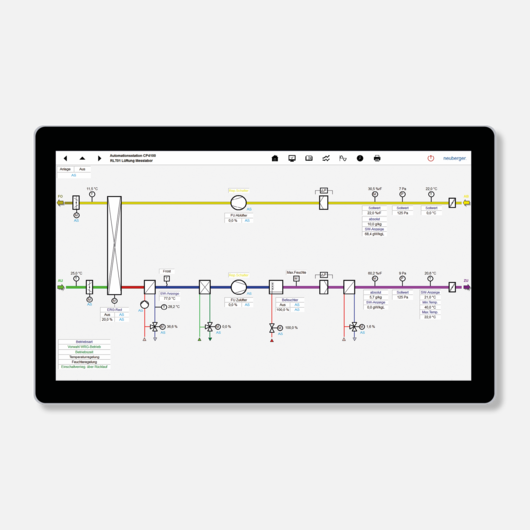
The slightest deviations in room conditions can have serious consequences. As a result, the efficient control and regulation of primary plants such as heating, ventilation and cooling is of the utmost importance in clean rooms and in the healthcare sector. The flexible PMC automation system combined with the powerful ProGrafNT building management system is the ideal solution for precise control of room conditions. In addition to controlling and monitoring stable ambient conditions using programmable logic controllers and ProGrafNT monitoring software, Neuberger also facilitates the evaluation and processing of quality-relevant data, which is another important tool for achieving GMP compliance in clean rooms.
Airlock control
Control of personnel and/or material airlocks to safeguard employees, products and the environment.
Regulation of room conditions
Regulation of all building services plants and a wide range of conditions in clean rooms.
Analyses and reports
Clearly presented evaluation of quality-relevant data.